- Home
- Contact Us
- News & Events
- Awards
- AAEES Awards Criteria
- 40 Under 40 Recognition Program
- Edward J.Cleary Award
- Excellence in Environmental Engineering and Science Education
- Gordon Maskew Fair Award
- Honorary Member
- International Honorary Member
- Ralph and Joe Bales Graber Science Award
- Stanley E. Kappe Award
- Environmental Communications Awards Competition
- Excellence in Environmental Engineering and Science Competition
- The AAEES Chapter Blue Marble Award
- Resources
- AAEES Microcredentials
- Annual Reports
- AAEES Press Releases
- AAEES Website How To VIdeos
- Environmental Engineer and Scientist
- Environmental Engineering Body of Knowledge
- PFAS Resources
- Specialty Examination Guide
- Students and Young Professionals Resources
- Who's Who in Environmental Engineering & Science®
- Leadership Opportunities
- Membership
- Donate
- Jobs
2019 Excellence in Environmental Engineering and Science® Awards Competition Winner
Superior Achievement in Environmental Engineering and ScienceCategory Entered: University ResearchEmergency Water Treatment with Ferrate(VI) in Response to Natural DisastersEntrant: Yang Deng, Ph.D., P.E. Entrant ProfileYang Deng, PhD, PE is Professor of Environmental Engineering at Montclair State University (the second largest National Research University in New Jersey) and a licensed environmental engineer (Florida). Prior to joining Montclair, he was a faculty at University of Puerto Rico. Prof. Deng earned his PhD (Environmental Engineering) at University of Miami and his BS and MS (Civil Engineering) from Tongji University (China). He is the recipient of 2018 Nanova Frontier Research Award (Chinese-American Professors in Environmental Engineering and Science). Prof. Deng has strong fundamental and applied research interests in innovative and sustainable water treatment technologies. He has secured funding (>$2 millions) from various sources. Through these projects, he mentored 5 postdocs, 11 graduate students, and 11 visiting professors, and authored/co-authored 110 peer-reviewed articles and 5 book chapters (cited 5,400+ times; h-index=35) over the past thirteen years. Revolutionizing water treatment with ferrate(VI) is his major research effort. His cumulative endeavors provides a scientific basis for utilization of ferrate(VI) for addressing different water challenges. The nominated emergency water treatment (EWT) project was inspired by the water crisis after Hurricane Maria in Puerto Rico. This represents the first scientific effort to apply ferrate(VI) to EWT for disaster-affected populations. He directed the research. Specific contributions include to design experiments, develop reactors, advise students, analyze data, prepare manuscripts, identify follow-on research directions, and prepare new proposals. Project findings was published as the Cover Paper of Environmental Science: Water Research & Technology. Others involved:
Project DescriptionComplexityFrequency and magnitude of natural disasters (e.g. hurricanes) have increased globally. United States was ranked No. 2 among the most frequently hit countries by natural disasters during 2006-2015. In 2017, Hurricanes Harvey, Irma, and Maria sequentially made landfalls in the U.S. and its territories, causing an economic loss of $170-300 billion and killing at least 164 American citizens. Clean water is a top priority after catastrophic disasters for drinking, cooking, and hygiene. However, three household emergency water treatment (EWT) methods FEMA recommends (i.e. boiling, chlorination, and distillation) are not all feasible or effective in many emergency situations. Recent Puerto Rican water crisis following Hurricane Maria highlights EWT research needs. One week after the storm, ~50% of 3.4 million residents did not have running water at 32°C. It was extremely difficult to transport water among different towns due to road damage, fuel shortage, and curfew. People started to collect drinking water from streams and runoff using unsafe containers (e.g. trash bins). The experience underlined distinctive challenges EWTs face:
An Integrated Approach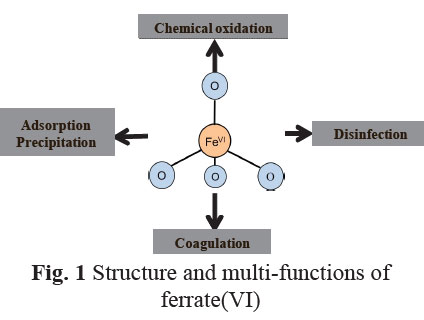
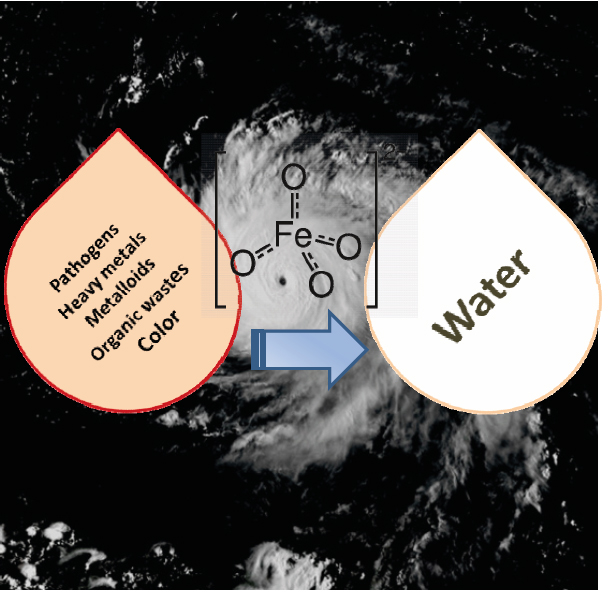
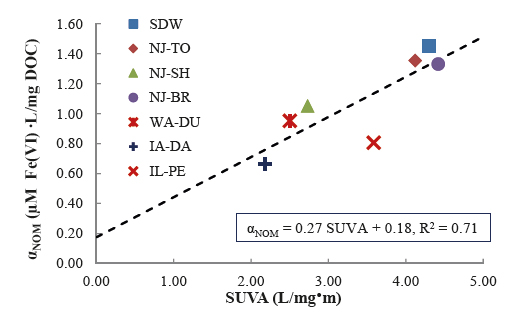
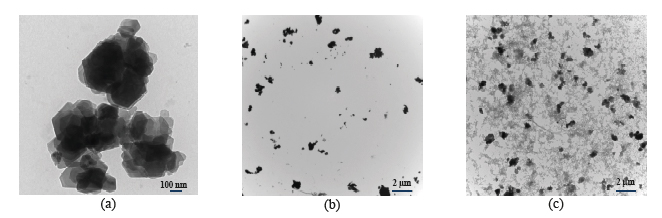
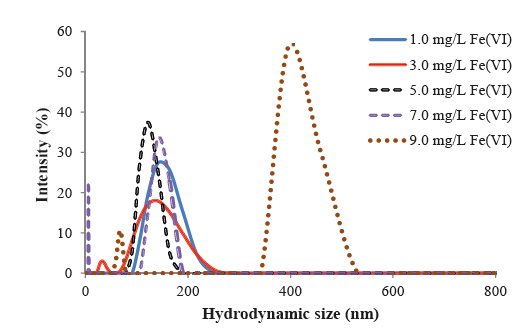
A holistic approach was adopted to develop and design innovative EWTs with ferrate(VI). The research was proposed and developed from PI's recent cumulative efforts in ferrate(VI) studies. Ferrate(VI), i.e. FeO42−(Fig.1), is a multi-function agent with little formation of disinfection byproducts (DBPs). The unique traits enables a straightforward but potentially effective solution to address aforementioned challenges. Six specific tasks, which tackled the challenges in different aspects, were pursued:
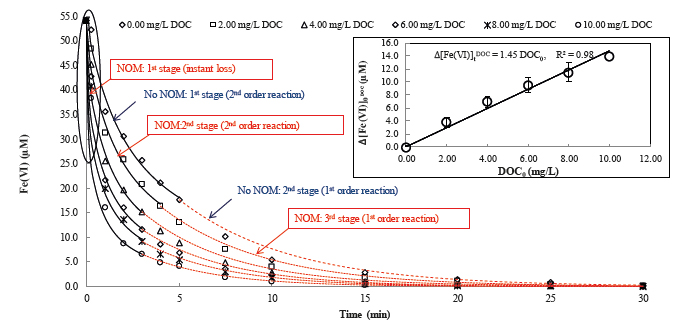
Quality and SignificanceClean water is essential during the aftermath of natural disasters. This study designed and developed a new, safe, resilient, affordable, and easy-to-use EWT, at a community or household scale, with ferrate(VI). The invented EWT can simultaneously treat multiple contaminants in disaster-polluted waters to meet with survival demands of disaster- affected populations and prevent infectious disease outbreaks. Moreover, the EWT is safe without production of DBPs. Iron sludge does not leach undesirable chemicals to pollute other environmental media during residual disposal. The residuals primarily comprising iron (hydr)oxides can be beneficially reused (e.g. stormwater treatment). Dr. Deng's work on ferrate(VI) has resulted in peer-reviewed publications[1]-[8] (including a journal cover paper[1]) and conference presentations. Significance of the research is also demonstrated in three enclosed testimonial letters. Originality and InnovationExisting EWT options difficultly tackle complex challenges under many emergency situations. Innovation of this project is the first application of ferrate(VI) chemistry knowledge to EWT. Of note, the novel EWT built on recent advances of ferrate(VI) science, which Dr. Deng has made cumulative contributions to, including:
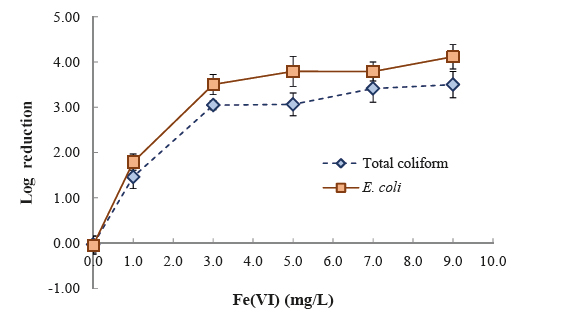
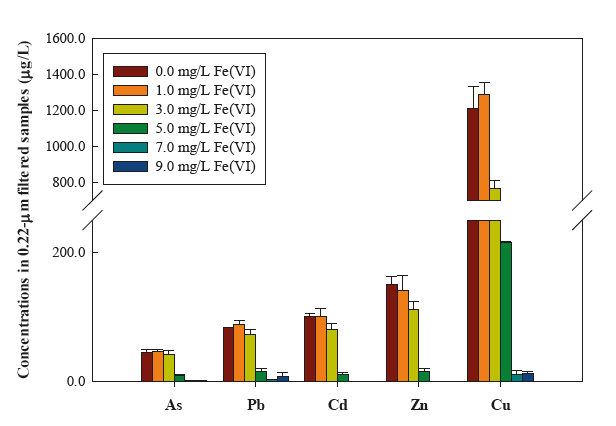
Multiple functions of ferrate(VI) enable simultaneous removals of a spectrum of contaminants. For example, ferrate(VI) (7.0 mg/L) can concurrently achieve 3.8 log reduction of total coliform, remove 98% of As (45 μg/L), 96% of Pb (84 μg/L), 95% of Cd (100 μg/L), 99% of Zn (145 μg/L), and 98% of Cu (1.2 mg/L), eliminate 86% of turbidity (11.88 NTU), and alleviate 60% of UV254 absorbance (0.247 cm-1) in a sewage-polluted water. Originality of this study is also embodied in the novel design of a household EWT (ferrate(VI) teabags, Photo 10). Simplicity, effectiveness, and low cost encourage more end users to accept the new EWT products. Economic and Social ImpactsThis project advances ferrate(VI) chemistry and develops new EWT technologies. Benefits are straightforward and immediate to disaster-affected populations, because clean water is vital to survival and public health. Sanitation with water can reduce the transmission of faceco-oral diseases and exposure to disease-bearing vectors. The resulted rapid disaster relief can save lives and minimize economic loss from disasters. Although this project targeted at EWT, the technologies can be readily applied at other scenarios such as industrial wastewater treatment (e.g. metal removal from flue-gas desulfurization (FGD) wastewater), small rural water systems, military bases, scientific expedition, and site remediation. References (PI's recent publications on ferrate(VI) studies)[1] Cui, J., L. Zheng, Y. Deng (Front Cover Paper) (2018) "Emergency Water Treatment with Ferrate(VI) in Response to Natural Disasters," Environmental Science: Water Research & Technology, 4, 339-470. [2] Deng, Y., C. Jung, Y. Liang, N. Goodey, T. Waite (2018) "Ferrate(VI) Decomposition in Water in the Presence of Natural Organic Matter (NOM)," Chemical Engineering Journal, 334, 2335-2342. [3] Lv, D., H. Zhang, L. Zheng, Y. Deng (2018) "Coagulation of Colloidal Particles with Ferrate(VI)," Environmental Science: Water Research & Technology, 4, 701-710. [4] Song, Y., Y. Deng, C. Jung (2016) "Mitigation and Degradation of Natural Organic Matter (NOM) during Ferrate(VI) Application for Drinking Water Treatment," Chemosphere, 146, 145-153. [5] Li, N., Y. Deng, D. Sarkar (2017) Ferrate(VI) Reaction With Effluent Organic Matter (EfOM) in Secondary Effluent for Water Reuse, in Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation, by Virender Sharma (Editor), ACS Publications. [6] Huang, X., Y. Deng, S. Liu, Y. Song, N. Li, J. Zhou (2016) "Formation of Bromate during Ferrate(VI) Oxidation of Bromide in Water," Chemosphere, 155, 528-533. [7] Zheng, L., Y. Deng (2016) "Settleability and Surface Characteristics of Ferrate(VI)-Induced Particles in Advanced Wastewater Treatment," Water Research, 93, 172-178. [8] Deng, Y., M. Wu, L. Zheng, H. Zhang, Acosta, H., Hsu, T. (2017) "Addressing Harmful Algal Blooms (HABs) Impacts with Ferrate(VI): Simultaneous Removal of Algal Cells and Toxins for Drinking Water Treatment," Chemosphere, 186, 757-761. Click images to enlarge in separate window. Click here to return to the list of 2019 winners. |